In Vivo Cellular Visualization



In vivo visualization of small Intestine
Micro-capillary and individual MHC-Class II:GFP+ cells are visualized by novel side-view endomicroscopy, Nature Methods, 7, pp. 303-305, Apr. 2010
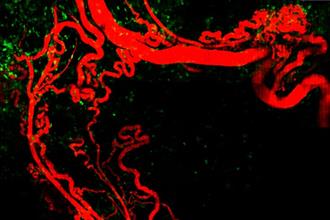
In vivo visualization of B16 melanoma in skin
Highly curled angiogeneic vasculature and infiltrated MHC-Class II:GFP+ cells including macrophages around B16 melanoma are visualized by custom-built in vivo microscopy setup.
Advances in these novel visualization technologies have allowed us to have a glimpse of numerous exciting processes those never have directly seen in vivo such as gene expression, regulation, protein activity, drug delivery, systemic cell trafficking / interaction, physiological response to external stimuli in the natural in vivo micro-environment. We're on the verge of a new era of micro-nano-scale in vivo visualization technologies as a novel tool for basic and translational biomedical research as well as a novel clinical imaging modality for diagnosis and monitoring for human care. Only with extensive interactions between multiple disciplines of physics, chemistry, biology, medicine and engineering, these visions can be accomplished.


Laser scanning microscopy
Customized video-rate laser scanning system with multiple laser lines.
Advanced In Vivo Cellular Imaging System
♦ Multi-modality in vivo laser scanning endomicroscopy
♦ In situ and in vivo endomicroscopic visualization of viscera and thoracic organs in animal
♦ In vivo nano-scale visualization / In vivo deep-tissue 3D visualization
♦ Label-free molecular profile visualization / High-throughput 3D visualization
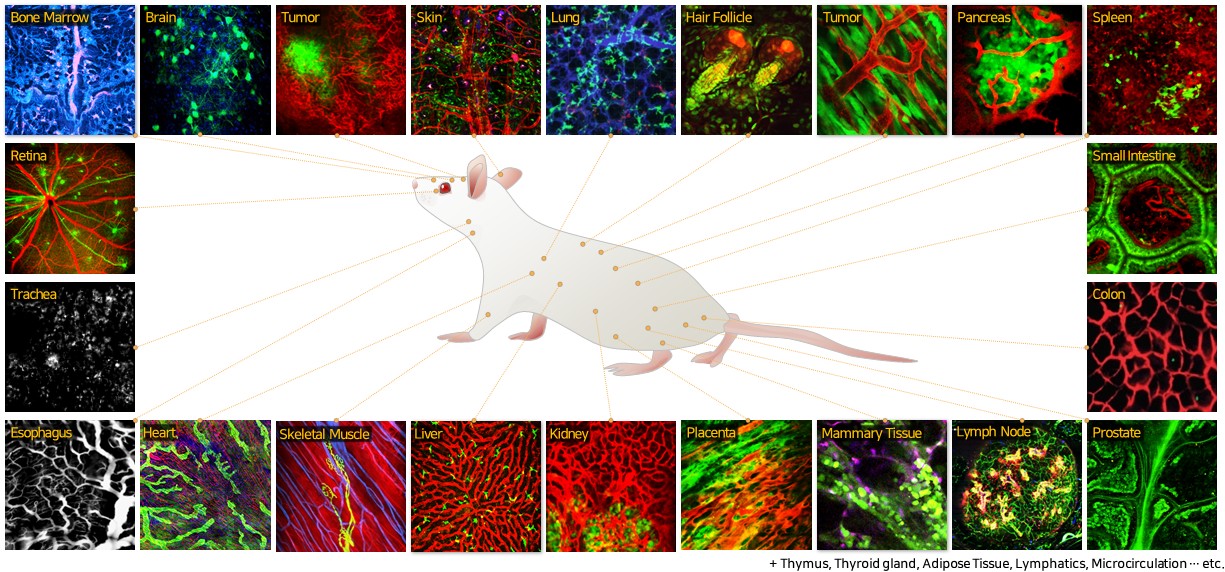
Systemic Cellular Visualization of Animal Model for Human Disease
♦ Visualization of dynamic lymphocyte trafficking in secondary lymphoid organs
♦ Monitoring of neural signaling at the cortex and hippocampus in brain
♦ Cellular visualization of the metastasized cancer micronodules at brain and liver
High-speed, Nano-scale Visualization of Organic and Inorganic Materials
♦ Ultrafast time-resolved visualization of nano-scale phenomena in advanced photonic material
Research Topics

Animal model, particularly mouse, has been an important test bed for basic and translational biomedical study preceding clinical application. Recent advances in genomic technology have allowed a creation of the animal model for human disease with a genetically encoded biomarker, notably green fluorescent protein (GFP) awarded Nobel Prize at 2008. Combined with a rapid development of fluorescent probes, including newly emerging nano-material, and mature molecular biology tools, it has opened up a new avenue to the observation of complex pathophysiology of human disease in the animal model with much greater detail at cellular and molecular level in high contrast and specificity. Accordingly, novel fluorescence imaging methods that can visualize anatomical structure with functional and molecular information provided by fluorescent probes in an animal model in vivo have drawn great attentions. While all of the major clinical imaging modalities such as ultrasound, CT, MRI and PET has been modified and adapted, optical imaging technologies, especially laser scanning confocal and multiphoton fluorescence microscopy, are the only one readily providing cellular resolution of sub-micrometer in a live animal. Over the recent years, these technologies enabled dynamic 3D visualization of the living specimen as various biological processes unfold in real-time, providing unprecedented insights those were impossible to obtain by traditional static 2D snapshots (i.e. histopathology). With the development of highly specific targeting agents and new endogenous contrast, potentially to be empowered by nano-technology, these new imaging methods have advanced to be directly applied to live animal in vivo, beyond conventional ex vivo thin tissue sections or in vitro cell cultures on Petri dish.
In Vivo Micro-Visualization Laboratory
Graduate School of Medical Science and Engineering, Korea Advanced Institute of Science and Technology (KAIST)
Rm.219, Basic Research Building (E6-6), 291 Daehak-ro, Yuseong, Daejeon, 34141, Republic of Korea
In Vivo Micro-Visualization Laboratory
Graduate School of Medical Science and Engineering (GSMSE)
Korea Advanced Institute of Science and Technology (KAIST)